Marc Dewey, Maria Siebes, Marc Kachelrieß, Klaus F. Kofoed, Pál Maurovich-Horvat, Konstantin Nikolaou, Wenjia Bai, Andreas Kofler, Robert Manka, Sebastian Kozerke, Amedeo Chiribiri, Tobias Schaeffter, Florian Michallek, Frank Bengel, Stephan Nekolla, Paul Knaapen, Mark Lubberink, Roxy Senior, Meng-Xing Tang, Jan J. Piek, Tim van de Hoef, Johannes Martens, Laura Schreiber & on behalf of the Quantitative Cardiac Imaging Study Group, “Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia“
Nature Reviews Cardiology volume 17, pages 427–450 (2020)
DOI: https://doi.org/10.1038/s41569-020-0341-8
Affiliation:
Marc Dewey, Andreas Kofler & Florian Michallek, with the Department of Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany
Marc Dewey, with the Berlin Institute of Health and DZHK (German Centre for Cardiovascular Research) Partner Site, Berlin, Germany
Maria Siebes, with the Department of Biomedical Engineering and Physics – Translational Physiology, Amsterdam University Medical Center, Amsterdam, Netherlands
Marc Kachelrieß, with the Division of X-Ray Imaging and CT, German Cancer Research Centre (DKFZ), Heidelberg, Germany
Klaus F. Kofoed, with the The Heart Centre Rigshospitalet, Department of Cardiology and Radiology, University of Copenhagen, Copenhagen, Denmark
Pál Maurovich-Horvat, with the MTA-SE Cardiovascular Imaging Research Group, Heart and Vascular Center, Semmelweis University, Budapest, Hungary
Konstantin Nikolaou, with the Universitätsklinikum Tübingen, Radiologische Klinik, Diagnostische und Interventionelle Radiologie, Tübingen, Germany
Wenjia Bai, with the Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, UK
Robert Manka, with the Institute of Diagnostic and Interventional Radiology and Department of Cardiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
Robert Manka & Sebastian Kozerke, with the Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Amedeo Chiribiri & Tobias Schaeffter, with the Department of Cardiovascular Imaging, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK
Tobias Schaeffter, with the Physikalisch-Technische Bundesanstalt, Medical Physics and Metrological Information Technologies, Berlin, Germany
Frank Bengel, with the Klinik für Nuklearmedizin, Medizinische Hochschule Hannover, Hannover, Germany
Stephan Nekolla, with the Nuklearmedizinische Klinik und Poliklinik, Klinikum rechts der Isar der TU München, DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany
Paul Knaapen, with the Department of Cardiology, VU University Medical Center, Amsterdam, Netherlands
Mark Lubberink, with the Department of Surgical Sciences – Nuclear Medicine & PET, Uppsala University, Uppsala, Sweden
Mark Lubberink, with the Medical Physics, Uppsala University Hospital, Uppsala, Sweden
Roxy Senior, with the Department of Cardiology, Royal Brompton Hospital London, London, UK
Meng-Xing Tang, with the Department of Bioengineering, Imperial College London, London, UK
Jan J. Piek & Tim van de Hoef, with the Heart Center, Amsterdam University Medical Center, Amsterdam, Netherlands
Johannes Martens & Laura Schreiber, with the Department of Cellular and Molecular Imaging, Comprehensive Heart Failure Center, Würzburg University Clinics, Würzburg, Germany
Abstract:
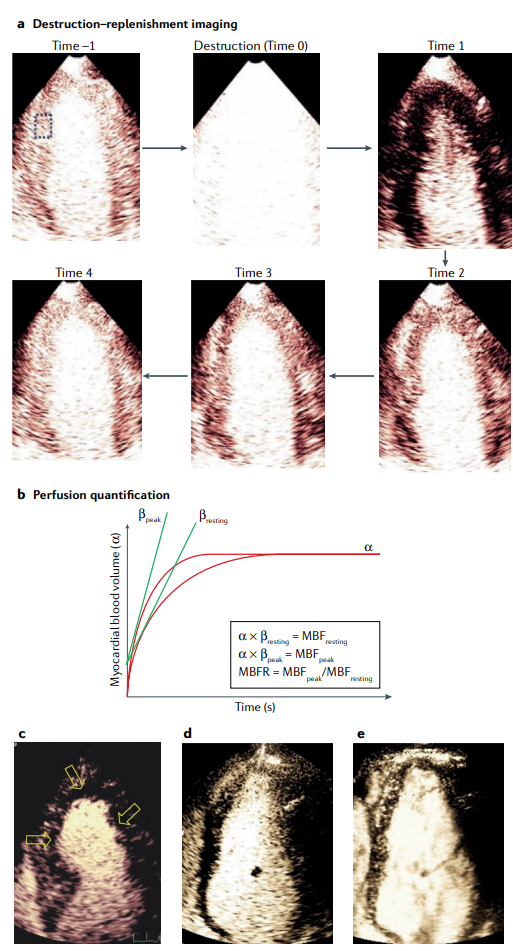
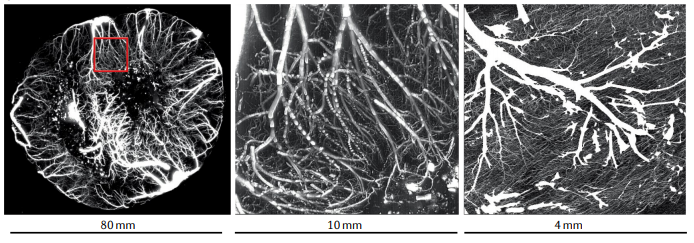
Cardiac imaging has a pivotal role in the prevention, diagnosis and treatment of ischaemic heart disease. SPECT is most commonly used for clinical myocardial perfusion imaging, whereas PET is the clinical reference standard for the quantification of myocardial perfusion. MRI does not involve exposure to ionizing radiation, similar to echocardiography, which can be performed at the bedside. CT perfusion imaging is not frequently used but CT offers coronary angiography data, and invasive catheter-based methods can measure coronary flow and pressure. Technical improvements to the quantification of pathophysiological parameters of myocardial ischaemia can be achieved. Clinical consensus recommendations on the appropriateness of each technique were derived following a European quantitative cardiac imaging meeting and using a real-time Delphi process. SPECT using new detectors allows the quantification of myocardial blood flow and is now also suited to patients with a high BMI. PET is well suited to patients with multivessel disease to confirm or exclude balanced ischaemia. MRI allows the evaluation of patients with complex disease who would benefit from imaging of function and fibrosis in addition to perfusion. Echocardiography remains the preferred technique for assessing ischaemia in bedside situations, whereas CT has the greatest value for combined quantification of stenosis and characterization of atherosclerosis in relation to myocardial ischaemia. In patients with a high probability of needing invasive treatment, invasive coronary flow and pressure measurement is well suited to guide treatment decisions. In this Consensus Statement, we summarize the strengths and weaknesses as well as the future technological potential of each imaging modality.
Supporting Bodies:
This work was funded by the UK EPSRC under grant EP/M011933/1 and the British Heart Foundation under grant PG/16/95/32350.